Special Review
- This function of WVU+kc is only available to Office of Sponsored Programs staff.
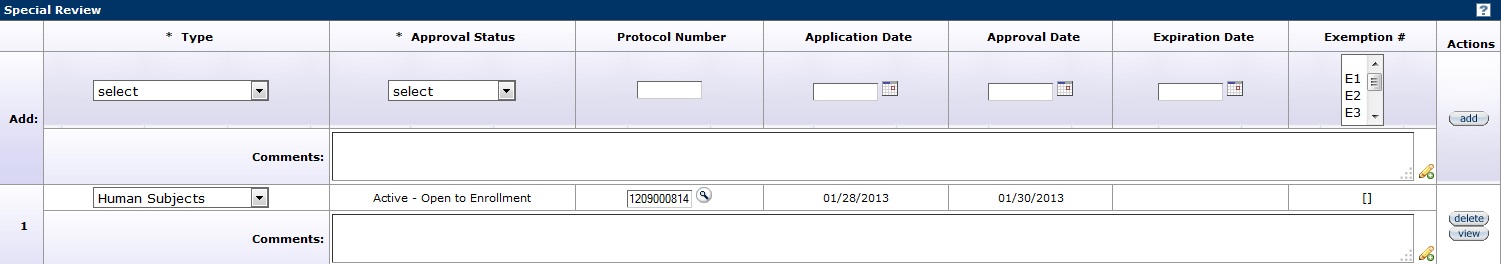
Figure 272 Institutional Proposal Document, Special Review Page – Special Review Section Example
The Special Review page contains information pertaining to reviews required for regulatory compliance. Types may be identified and their approval status is recorded along with the relevant protocol number, key information, and any comments provided by reviewers. A special review refers to a grant proposal that requires additional review by an institutional committee or official, for example, proposals that include the use of Biohazard Materials in research.
Field |
Description |
|---|---|
Type |
Required. The type of special review relevant to the document. Click on the drop-down
|
Approval Status |
Required. The status of the special review type. Click on the drop-down
|
Protocol Number |
The unique identifier assigned to the protocol submitted for regulatory review. The protocol # is the number assigned to the protocol submitted by the PI to WVU’s relevant committee (e.g., IRB, IACUC). A unique number assigned by the institution and/or compliance committee to a specific research protocol. Search for the protocol number for any human subject research protocols by clicking the search
|
Application Date |
The date the PI submitted a protocol/request for permission to the relevant regulatory committee for special review. If known, enter the date in the text box in MM/DD/YYYY format or select the date by clicking on the calendar |
Approval Date |
The date the protocol/request for permission was approved by the relevant regulatory committee for special review. If known, enter the date in the text box in MM/DD/YYYY format or select the date by clicking on the calendar |
Expiration Date |
The date an existing protocol expires. If known, enter the date in the text box in MM/DD/YYYY format or select the date by clicking on the calendar |
Exemption # |
One of six (6) categories of exempt research specified by the Common Rule (45 CFR 46.101(b)). If the proposed project is exempt, an exemption number is generally issued and should be entered into this field. Select the appropriate exempt number from the spin box. |
Comments |
Type within the text box to enter internal note relevant to the protocol or special review item. |
Table 94 Institutional Proposal Document, Special Review Page – Special Review Section Column Descriptions
Add Special Review Lines
The Type and Approval Status are required fields to add a special review to a proposal. Enter all information known about the protocol and comments regarding the special review. After all selections have been made, click the add ![]() button to add the review entry as a numbered line item. To remove a line item, click the delete
button to add the review entry as a numbered line item. To remove a line item, click the delete ![]() button.
button.
Searching Protocols
To search for human subject research protocols within WVU+kc, click the search ![]() icon in the Protocol Number to use the Protocol Lookup feature. Enter information within the search fields provided. If searching for all protocols submitted by a particular investigator, it may be useful to utilize the wildcard search by entering the investigator’s last name in the Investigator field and searching. For example, if searching for a protocol submitted by Dr. Bluebeard, enter *bluebeard* in the Investigator field and all of Dr. Bluebeard’s protocols will be displayed in the search results. Refine the search for Dr. Bluebeard if the type of protocol is known, by selecting a type in the Protocol Type field such as NHSR, Exempt, Expedited, or Full Board.
icon in the Protocol Number to use the Protocol Lookup feature. Enter information within the search fields provided. If searching for all protocols submitted by a particular investigator, it may be useful to utilize the wildcard search by entering the investigator’s last name in the Investigator field and searching. For example, if searching for a protocol submitted by Dr. Bluebeard, enter *bluebeard* in the Investigator field and all of Dr. Bluebeard’s protocols will be displayed in the search results. Refine the search for Dr. Bluebeard if the type of protocol is known, by selecting a type in the Protocol Type field such as NHSR, Exempt, Expedited, or Full Board.
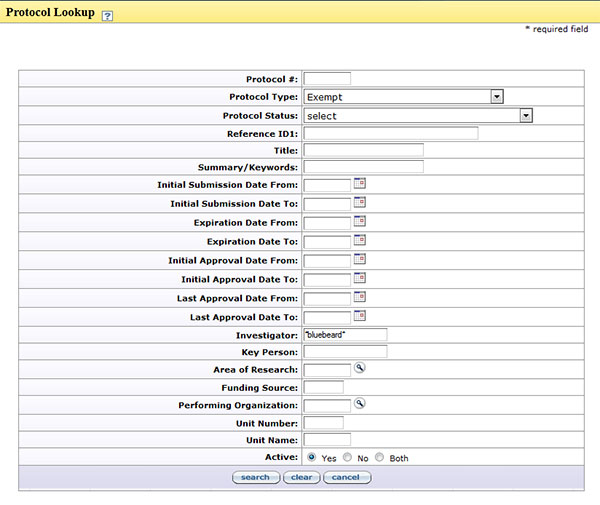
Figure 273 Institutional Proposal Document– Protocol Lookup Page