Create Renewal with Amendment
The WVU IRB will conduct a continuing review of ongoing research at intervals that are appropriate to the level of risk for each research protocol, but not less than once per year. Continuing review must occur as long as the research remains active for long-term follow-up of patients, even with the research is permanently closed to the enrollment of new participants and all participants have completed all research-related interventions. Continuing review of research must occur even when the remaining research activities are limited to the analysis of private identifiable information.
The approval date and approval expiration date are clearly noted on all IRB certifications sent to the PI and must be strictly adhered to. Investigators should allow sufficient time for development and review of renewal submissions. Notifications will be sent to investigators to remind of renewals; however, it is the investigator’s responsibility to ensure that the continuing review of ongoing research is approved prior to the expiration date.
- Continuing review and re-approval must occur by midnight of the date when IRB approval expires. By federal regulation, no extension to that date can be granted.
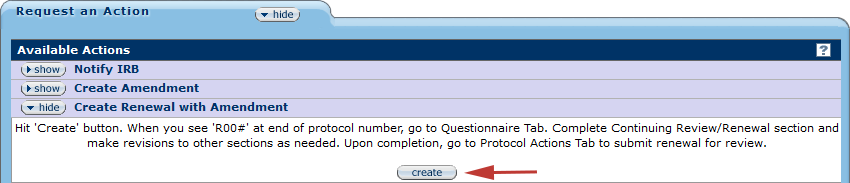
Figure345 Protocol Document, Protocol Actions Page – Request an Action Section, Create Renewal with Amendment
Click the create button and a new protocol document will be opened. All questions regarding the renewal should be completed on the Questionnaire page.
Submit a Renewal with Amendment
Navigate to the Submit for Review subsection of the Request an Action section.

Figure 346 Protocol Document, Protocol Actions Page – Request an Action Section, Submit Continuing Review with Amendment
Select “Continuing Review/Continuation with Amendment” from the drop-down menu in the Submission Type field. The protocol type will be autoselected from the drop-down menu in the Submission Review Type field. Click the submit ![]() button to submit the renewal.
button to submit the renewal.
- While working on a renewal, your protocol number will have a suffix beginning with R, such as 1401126339R001. This suffix indicates that the protocol is a renewal. The status "Renewal in Progress" means that the renewal is currently being edited for submission and HAS NOT been submitted. the status "Routing Protocol" means the renewal has been submitted and is being reviewed. Once the renewal is approved by the IRB, the suffix on the protocol will no longer appear.