Watch Video
- Protocol Training Video
Click arrows in the upper-left corner of video to navigate by section.
Reference Guides
Protocol
The protocol document is an e-doc that works as an electronic protocol application form created by a researcher (or an administrator) that is submitted to West Virginia University’s Institutional Review Board (IRB) via the Office of Research Integrity and Compliance. The document will track required revisions, approval amendments, renewals, and expirations. It is designed to take into consideration federally mandated criteria as well as WVU’s institutional policies regarding research involving human participants in order for the IRB to assess the risk/benefit ratio for the proposed research project.
In this topic:
|
Jump to page subtopics: |
Preface
It is important to have a basic understanding of the context of the protocol document’s use and context of such use before you begin to use it. It is also helpful to understand the trigger or circumstances that initiate the need to use the functions of the protocol document along with the fundamentals of its life cycle.
Business Needs and Purpose
The protocol document is completed whenever a researcher:
- Wants to submit a protocol application to the WVU IRB;
- Needs to revise, amend, or renew a protocol; or
- Needs to notify the IRB of an issue or request a change to the status of the protocol.
The protocol document is used to:
- Ensure compliance with WVU research requirements, or to
- Obtain Human Subjects research approval in order for a sponsored award to be approved.
Policy
WVU has policies that drive the procedures and rules associated with usage of the protocol document including:
- Compliance with federal government regulations and related business rules
- 45 CFR 46.107 – Institutional Review Boards
- 45 CFR 164.512(i)(1)(B) – Privacy Boards
- 21 CFR 50 and 56 – FDA Protection of Human Subjects
- Conflict of Interest
- For more information about WVU’s policies, see the ORIC’s Standard Operating Procedures manual.
Permitted Actions by Role
There are various roles that can be assigned to users on each protocol. These roles will provide various levels of access to a protocol from view-only to being allowed to edit the information within the protocol document. By default, users with the Protocol Creator role are permitted to initiate a new protocol document. This initiator is automatically granted an Aggregator role for the protocol document preparation and this user’s name is displayed in the Aggregator field of the Assigned Roles section.
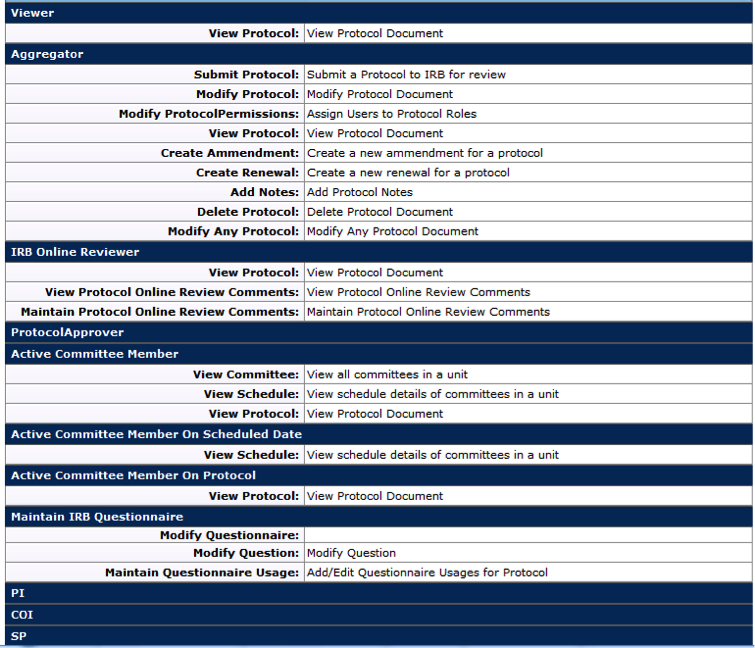
- For more information about assigned user roles, see Permissions in the Protocol section.
Process Flow
Protocol Document Review & Approval
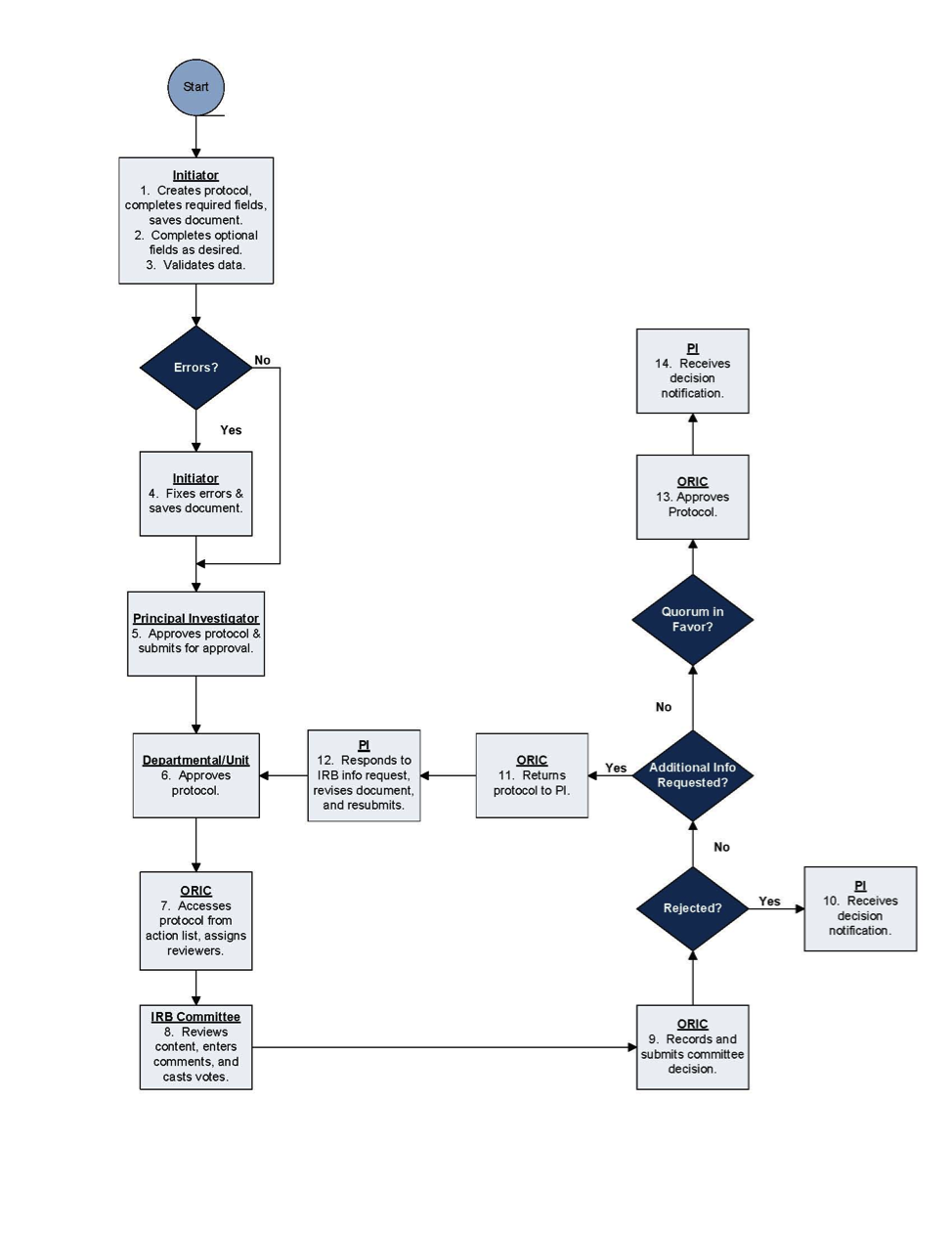
Figure 137 Simplified Protocol Document Workflow – Process Flow Chart Diagram
Status
Document-specific status generally relates to completion-related actions, which are the states of the document while it is in the process of being prepared prior to submission for approval. Examples of a protocol document’s status types are:
- Active-Open to Enrollment indicates the protocol has been approved by the IRB and is currently active.
- Active-Closed to Enrollment indicates the protocol has been approved by the IRB and is currently active, but is closed to accrual of subjects.
- Amendment in Progress, Renewal in Progress indicates that an amendment or renewal has been submitted for the protocol but has not been approved.
- Closed Administratively indicates the protocol has been closed and is no longer active.
- Exempt-Acknowledged indicates the Exempt research has been approved.
- Expired indicates that the protocol has expired and is no longer active.
- IRB Review Not Required/Not Human Subject Research indicates that the protocol has is a non-human subject research project and does not require review by the IRB.
- Pending/In Progress indicates that the protocol is currently under development and has not been submitted to the IRB for review.
- Recalled in Routing indicates that a protocol was submitted and was in the process of obtaining approval before reaching the ORIC, but was withdrawn by the PI or an aggregator for additional revisions. If a protocol is recalled in routing for edits, it will have to receive all appropriate approvals again, once resubmitted.
- Submitted to IRB indicates the protocol has been submitted to the IRB but has not been reviewed.
- Specific Minor Revisions Required or Substantive Revisions Required indicate the protocol was submitted to the IRB, reviewed, and returned to the PI to make revisions.
Workflow Status
Workflow status generally relates to approval-related actions, which are the routing of the completed document in the system for approval. Even after approval and final disposition actions are completed, a permanent historical record of the protocol document is always accessible.
To view an e-doc’s route log:
Click ![]() button >
button > ![]() link > Log column
link > Log column ![]() icon
icon
This allows users to review Actions Taken and the Route Status for the document.
- For more information about Workflow (routing) Status changes, see Route Status in Routing Rules of the Overview section.
Access
Access to the protocol document is based on groups, roles, permissions, and responsibilities that are defined in the Kuali Identity Management (KIM) module. Generally speaking, the KC user interface allows researchers to create new protocol documents, while unit and central admin users are able to search for and edit them.
- For more information about e-doc access, see Access in the Overview section.
Creating a New Protocol Document
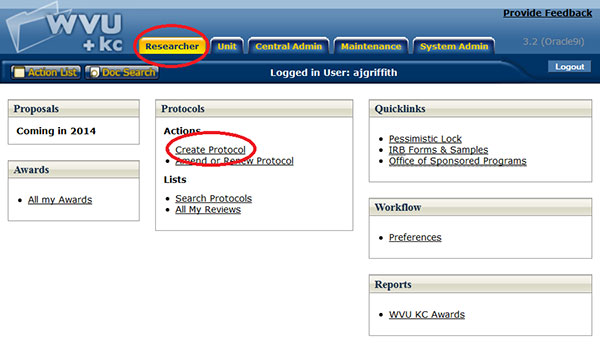
Figure 138 Create Protocol from the Researcher Menu
A protocol e-doc may only be created by an authorized person with the Create Protocol role in KC. This is done by clicking the Create Protocol link in the Actions section of the Protocols group on the Researcher menu.
- For more information about creating a new e-doc, see Initiating a Document in the Common E-Doc Operations section.
Accessing an Existing Protocol Document
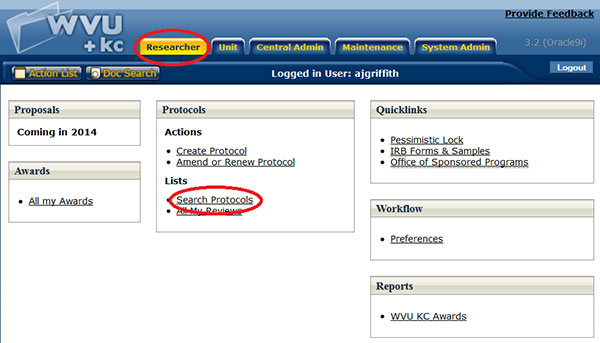
Figure 139 Search Protocols from the Researcher menu
An existing protocol e-doc may be accessed by researcher by clicking the Search Protocols link in the Lists section of the Protocols group on the Researcher menu which will initiate the Protocol Lookup page. The lookup function allows users to search reference table information from maintenance documents and display a list of valid values from which to select and return.
Action List & Doc Search
An existing protocol document may also be accessed via the ![]() or
or ![]() global buttons at the top, left of the WVU+kc screen. Protocols can be accessed through the Action List by clicking on the Id number for a protocol. Protocols can be accessed through the Doc Search function by enabling the Document Lookup page and searching for the document. Search criteria fields allow users to select and/or enter criteria and then click a search button to produce a results table. From the results table, users can click a link to directly open the document.
global buttons at the top, left of the WVU+kc screen. Protocols can be accessed through the Action List by clicking on the Id number for a protocol. Protocols can be accessed through the Doc Search function by enabling the Document Lookup page and searching for the document. Search criteria fields allow users to select and/or enter criteria and then click a search button to produce a results table. From the results table, users can click a link to directly open the document.
- For more information about using the
 , see Using the Action List in the Common E-Doc Operations section.
, see Using the Action List in the Common E-Doc Operations section. - For more information about using the
 , see Searching for a Document and Doc Search in the Common E-Doc Operations section.
, see Searching for a Document and Doc Search in the Common E-Doc Operations section.
Document Layout
The protocol document is comprised of a header at the top, right; seven tabbed pages (Protocol, Personnel, Questionnaire, Permissions, Notes & Attachments, Protocol Actions, Medusa, and sometimes Online Review) organized horizontally across the top; a page body area that dynamically displays content based on the currently-accessed page; tabbed, expandable/collapsible sections containing fields on each page; and an action buttons area at the bottom, center of any page.
| Major Document Component | Description |
|---|---|
| Header Area | Document identification information at the top, right of the document containing both common and document-specific fields. |
| Tabbed Pages | Pages that make up the document which are accessible by clicking the folder tab for each. These are groupings of functionally related information for the purpose of display and collection and are generally designed to be completed in left-to-right order. |
| Tabbed Page Sections | Multiple tabbed sections of each page containing data entry/selection/display fields that are expandable/collapsible via hide/show buttons. Some sections contain subsections-the labels for which are highlighted in a darker shade of grey. |
| Action Buttons | Buttons that appear at the bottom, center of the document (regardless of the page), some of which are common to all e-docs and some of which are unique to the particular document. |
- For more information about e-doc component features that are common to all e-docs, see E-Doc Topology in the E-Doc Fundamentals section of the Overview.
- For more information about e-doc component features that are unique to the protocol e-doc, scroll or click to navigate to the subsequent headings for each page and section that follows below.
Document Header

Figure 140 Protocol Document Header Example
The document header area of the protocol document is comprised of six display-only fields at the top, right of the document. This is viewable regardless of the currently –accessed page and contains a minimum amount of basic information about the protocol document to allow users to be certain the correct document has been accessed. Some fields are populated upon creation, while other display information only after subsequent user action. Each is described as follows.
| Field | Description |
|---|---|
| Document Id | The system-generated number that uniquely identifies the currently-displayed protocol document. This number is assigned upon initiation of the document.
|
| Initiator | The User ID of the logged-in user who first created the new protocol document and is displayed when a new protocol is created. This ID also serves as a link to additional information about the user. |
| Protocol# | This number is automatically assigned by the system upon save of the document after the required fields on the Protocol page have been completed.
|
Status |
The state the protocol document is in. Note that the status is different from the workflow routing status. This field will also indicate the review type the protocol received. |
| Last Updated | The time and date is displayed after the first save of the document and is updated with each save thereafter. |
| Expiration Date | The last date of the current approval period and is generated by an approval action. This date can change due to any renewal approval actions. |
Table 51 Protocol Document Header Field Descriptions
The protocol document is made up of six pages, each of which is accessed by clicking its corresponding tab. Upon initiation, the default view is the Protocol page. Each page is labeled according to the type of information collected and contains tabbed, expandable/collapsible sections
- Page Order & Completion: The pages in WVU+kc e-docs are designed with the intent that users complete them in left-to-right order, although this is not required. In general, users may choose to work on any page in any order to complete the document. The one caveat is that users must first fill out the required fields on the Protocol page and click
 in order to proceed to any other page.
in order to proceed to any other page.

Figure 141 Protocol Document Horizontal Page Tabs Layout
| Page Name & Topic Link | Description |
|---|---|
| Protocol | General information about the protocol. |
| Personnel | The people involved in doing the work for the research project. |
| Questionnaire | The protocol questions themselves and selection/entry tools for answering them. |
| Permissions | Assign roles to users indicating who can do what with regard to completion of the protocol document. |
| Notes & Attachments | Add textual comments or documents related to the protocol or personnel information. |
| Perform tasks via commands such as submit, notify, expire, validate, print, copy, route, etc. |
Action Buttons
Standard ![]() and
and ![]() action buttons appear at the bottom, center of the screen, regardless of currently-accessed page.
action buttons appear at the bottom, center of the screen, regardless of currently-accessed page.
Additional action buttons appear according to the currently-accessed page, the logged-in user’s permissions, the document passing through various stages of completion, and/or the document passing through various stages of approval routing including:
![]()
![]()
![]()
![]()
- For more information about action buttons, see Action Buttons in Selection, Entry, & Action Tools section.
Subtopics:
- Protocol
- Personnel
- Questionnaire
- Permissions
- Notes & Attachments
- Protocol Actions
- Data Validation
- Summary & History
- Copy to New Document
- Route Log
- Online Review