Protocol
Watch Video
- Protocol Training Video
Click arrows in the upper-left corner of video to navigate by section.
Reference Guides
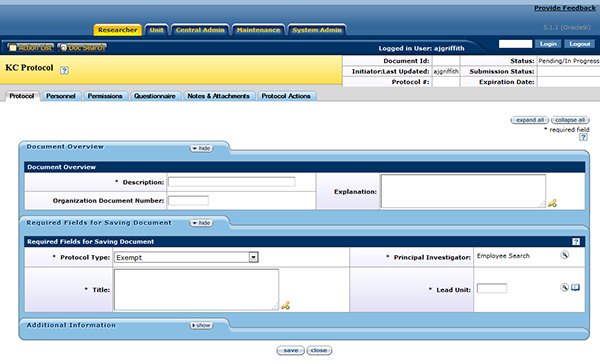
Figure 142 Protocol Page
The Protocol page is by default the first page displayed upon creating a new protocol document or accessing an existing one. An entire protocol document is comprised of seven pages: Protocol, Personnel, Questionnaire, Permissions, Notes & Attachments, Protocol Actions, and Medusa. By default, the Protocol page is the first page displayed when either creating a protocol or accessing a saved one. There are three tabbed sections located within the Protocol page: Document Overview, Required Fields for Saving Document, and Additional Information. These sections collect general information fundamental to the protocol such as title, Principal Investigator (PI), funding source, and protocol participants.
| Section Name & Topic Link | Description |
|---|---|
| Document Overview | General information. |
| Required Fields for Saving Document | Minimum identifying information. |
| Additional Information | Attach additional reference numbers such as FDA IND or IDE numbers. |
Table 52 Protocol Document, Protocol Page – Section Descriptions
Required Fields for Saving Protocol
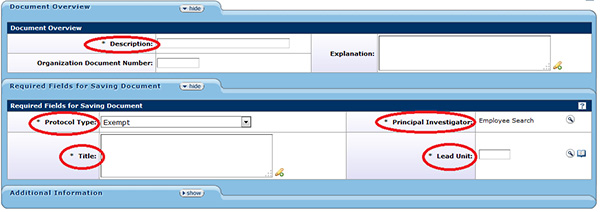
Figure 143 Protocol Document, Protocol Page – Required Fields for Saving Document
There are five fields on the Protocol page that are required to be completed before the protocol can be saved and being able to move on to another page.
| Field | Section | Description |
|---|---|---|
| Description | Document Overview |
The text entered into this field should be a short title to describe the project. The limit for this field is 40 characters. |
| Protocol Type | Required Fields for Saving Document | Select the appropriate review type for the protocol from the eight (8) options in the drop-down
|
| Title | Required Fields for Saving Document | Enter the full title for the protocol by clicking within the text box and entering the required text or clicking the add note |
| Principal Investigator | Required Fields for Saving Document | Indicate the person who will have absolute responsibility for the overall conduct of the research by clicking the search |
| Lead Unit (Department) |
Required Fields for Saving Document | Indicate the department that is the primary home of the PI. Click the search |
Table 53 Protocol Document, Protocol Page – Required Fields for Saving Document Descriptions
Subtopics