Required Fields for Saving Document
Watch Video
- Protocol Training Video
Click arrows in the upper-left corner of video to navigate by section.
Reference Guides
There are four fields located within the Required Fields for Saving Documents section on the Protocol page that must be completed before a protocol document can be saved. These fields collect a minimum amount of basic information to uniquely identify the new document in the WVU+kc system.
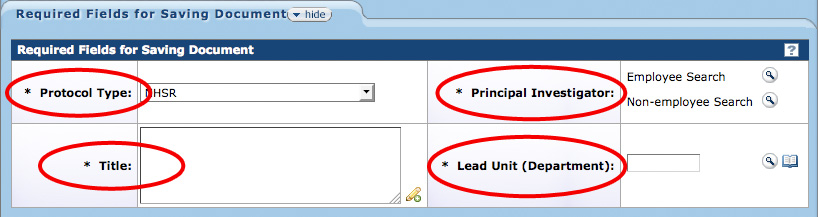
Figure 145 Protocol Document, Protocol Page – Required Fields for Saving Document Section
Protocol Type
Select the intended use of the protocol from the eight (8) choices in the drop-down ![]() menu. The
default type is Exempt. The selected type will imply the level of review
the protocol will likely require; however, the final determination will be made by the ORIC upon review
of the submitted protocol.
menu. The
default type is Exempt. The selected type will imply the level of review
the protocol will likely require; however, the final determination will be made by the ORIC upon review
of the submitted protocol.
- The Protocol Type selection will also determine the questions that users will be required to answer on the Questionnaire page of the protocol document. Selecting the incorrect review type may result in answering more questions than needed or not answering enough questions, in which case the ORIC office will return your protocol and request additional information. If you need assistance determining the type of protocol you are submitting, please review the Human Subject Regulations Decision Charts.
| Protocol Type | Description |
|---|---|
| Central IRB | A Central Institutional Review Board (CIRB) review allows an investigator to enroll patients into adult and pediatric Cooperative Group clinical trials significantly faster than when using the traditional IRB review method. |
| Clinical Trials (External Protocol Only) | This selection should only be made if you are conducting an industry-sponsored clinical trial that already has an external protocol associate with it. If you are conducting a clinical trial that does not have an existing external protocol, you should select "Full Board" as your protocol type. |
| Emergency Use | Emergency Use allows the use of an investigational drug or biological product with a human subject in a life-threatening situation in which no standard acceptable treatment is available and in which there is not sufficient time to obtain IRB approval. |
| Exempt | Exempt protocols involve research with very minimal or no risk; in general, research that does not propose to disrupt or manipulate the normal life experiences of subjects, incorporate any form of intrusive procedures, or involve deception. |
| Expedited | Expedited reviews are conducted by one or more members of the IRB using the same criteria as the full IRB. Research activities eligible for expedited review do not present no more than minimal risk to human subjects and involve only procedures listed in the Federal Register. |
| Full Board | Full Board review is required for human subject research that is not classified as exempt or expedited. |
| Humanitarian Use Device (HUD) | The HUD program creates an alternative pathway for getting market approval for medical devices that may help people with rare diseases or conditions. Applicants must provide a description of the disease/condition, indications for the use of the device, and reasons why such a device is needed for the patient population. |
| Not-Human Subject Research (NHSR) | NHSR is a document that provides assurances your study does not need to be approved by the IRB. If you answer "No" to any of the questions in the Human Subjects Research section of the Questionnaire page, your research may qualify for NHSR. If you choose NHSR as your protocol type and answered "Yes" to any question in the Human Subjects Research section, you should contact the ORIC office at 304-293-7073 to determine your correct protocol type. |
Table 55 Protocol Document, Protocol Page – Required Fields for Saving Document Section, Protocol Type Descriptions
Title
Create a title for the protocol by clicking within the text box and entering the required text. You may click the add note ![]() icon to view/edit/paste text in a new browser window. Click the
icon to view/edit/paste text in a new browser window. Click the ![]() button to return to the text entry field in the document. This field is limited to 2,000 characters. After save, click the green arrow
button to return to the text entry field in the document. This field is limited to 2,000 characters. After save, click the green arrow ![]() symbol to view full text in a separate browser window.
symbol to view full text in a separate browser window.
Principal Investigator
Indicate the person who will have absolute responsibility for the overall conduct of the research by
clicking the search ![]() icon to search personnel. Once the PI has been located in the search results,
return the value to add the designated person to the protocol. This selection will become read-only
after saving; however, a Co-I can be changed to a PI on the Personnel page by clicking on the detailed
information section of that particular person.
icon to search personnel. Once the PI has been located in the search results,
return the value to add the designated person to the protocol. This selection will become read-only
after saving; however, a Co-I can be changed to a PI on the Personnel page by clicking on the detailed
information section of that particular person.
- PIs must be faculty or staff members with organization-paid appointments. Doctoral or graduate students’ research must be supervised by a faculty member who would be indicated as the PI on the project with the student serving as Co-Investigator.
Department / Lead Unit
Indicate the department that is the primary home of the PI on the protocol. Click the search ![]() icon to search available WVU units. This selection becomes read-only after saving. Additional units may be specified in the Unit Details of the PI located on the Personnel page.
icon to search available WVU units. This selection becomes read-only after saving. Additional units may be specified in the Unit Details of the PI located on the Personnel page.
- For more information about initiating a document, see Initiating a Document in the Common E-Doc Procedures section.
- For more information about saving a document, see Saving a Document in the Common E-Doc Procedures section.
- For more information about searching for a saved document, see Searching for a Document in Common E-Doc Procedures.