Request an Action
Watch Video
- Protocol Training Video
Click arrows in the upper-left corner of video to navigate by section.
Reference Guides
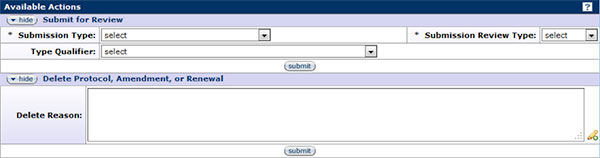
Figure 196 Protocol Document, Protocol Actions Page – Request an Action Section
The Request an Action subsection of the Protocol Actions page displays information for each available action. Available actions differ depending on factors such as previously-performed actions, pending actions requests, the role of the logged-in user, and document status. When working with a new protocol document (with the status of pending/in progress), the two (2) available actions are: Submit for Review and Delete Protocol, Amendment, or Renewal.
Submit for Review
There are two required fields in this subsection: Submission Type and Submission Review Type. Select the appropriate Submission Type from the drop-down ![]() menu. For new protocols, the only option available is Initial Protocol Application for Approval. The appropriate Submission Review Type will automatically be selected from the drop-down
menu. For new protocols, the only option available is Initial Protocol Application for Approval. The appropriate Submission Review Type will automatically be selected from the drop-down ![]() menu that corresponds with the protocol type selected on the Protocol page: Full, Expedited, Exempt, or NHSR. Click the submit
menu that corresponds with the protocol type selected on the Protocol page: Full, Expedited, Exempt, or NHSR. Click the submit ![]() button to officially submit the protocol document for review.
button to officially submit the protocol document for review.
Delete Protocol, Amendment, or Renewal
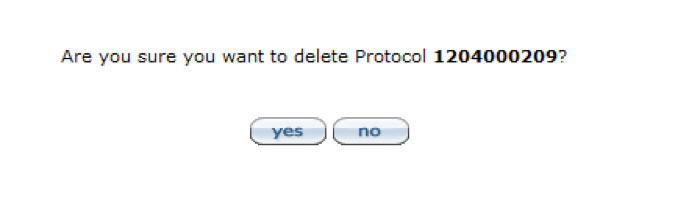
Figure 197 Protocol Document, Protocol Actions Page – Request an Action Section, Delete Protocol, Amendment or Renewal
If at anytime, a protocol, amendment, or renewal would need to be deleted, use the Delete Protocol, Amendment, or Renewal action. Click within the text box and enter the text to provide an explanation for the deletion then click the submit ![]() button to officially record the reason entered for the deletion. The system will make you confirm that you want to delete the particular protocol in the message. Amendments will end with A- suffix, such as A001, and renewals will end with R-suffix, such as R001. If deleting an amendment or renewal, you will only delete the amendment or renewal, not the original protocol. Click the yes
button to officially record the reason entered for the deletion. The system will make you confirm that you want to delete the particular protocol in the message. Amendments will end with A- suffix, such as A001, and renewals will end with R-suffix, such as R001. If deleting an amendment or renewal, you will only delete the amendment or renewal, not the original protocol. Click the yes ![]() button to confirm the deletion.
button to confirm the deletion.
- The delete action is only be available for amendments/renewals that have not been submitted to the Office of Research Integrity & Compliance.
Available/Unavailable Actions
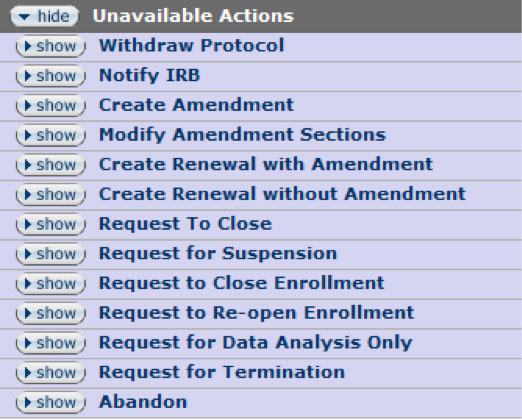
Figure 198 Protocol Document, Protocol Actions Page – Request an Action Section, Unavailable Actions
Depending on your role, previously-requested actions, and the current document status, the list of available/unavailable actions will change dynamically. To see a list of the protocol actions that are currently unavailable, click on the show ![]() button located beside the Unavailable Actions tab. The list of available actions changes dynamically based on the role of the user and the current state of the protocol document. For example, the PI will see a different set of actions than an IRB administrator. Additionally, there will be a different set of actions available before and after the protocol has been approved. The following is a list of various available actions that are possible for a PI within the system.
button located beside the Unavailable Actions tab. The list of available actions changes dynamically based on the role of the user and the current state of the protocol document. For example, the PI will see a different set of actions than an IRB administrator. Additionally, there will be a different set of actions available before and after the protocol has been approved. The following is a list of various available actions that are possible for a PI within the system.
- Follow-up actions appear in the Request an Action section after certain actions are performed. Only those actions which can logically be performed given the new state of the protocol document dynamically appear in the refreshed list of available actions. The logged-in user’s role and associated permissions also dictate the actions that are available.
| Actions | Description |
|---|---|
| Create Amendment | An amendment is a request by the PI to make a change to a previously approved protocol. This change may be procedural or informational. A frequently requested amendment is a change to the personnel working on the project. For an amendment, WVU+kc will allow changes to the data elements in the protocol in the specific sections identified by the researchers.
|
| Create Renewal with Amendment | A renewal (also referred to as a continuation) is a request (usually annually) to continue work on a previously approved project. A renewal with an amendment is used if it is necessary to modify information within an approved protocol at the time of its continuing review. The acceptance of the renewal by the IRB committee will generally extend the expiration date.
|
| Delete Protocol, Amendment, or Renewal | Select this action to delete an unsubmitted protocol from the system. |
| Notify IRB | Select this action to inform the IRB of an event or provide new information regarding an approved protocol, such as an adverse event, UPIRTSO, or deviation/exception/violation. Attach any relevant information.
|
| Request for Suspension | This function is currently not available in the WVU+kc system. If you feel the need to suspend your study, contact the ORIC immediately to discuss your project. |
| Request to Close | Submit a request to close the study with a comment. This action creates a protocol submission record. A closure form must be attached when submitting a Request to Close.
|
| Request to Close Enrollment | Submit a request to close enrollment on an active protocol.
|
| Submit for Review | Submit a new protocol, amendment, renewal, or response to IRB office for review. |
| Withdraw Protocol | After a protocol has been submitted, approved by departments, and received by the Office of Research Integrity & Compliance, the PI can request to withdraw it before it is reviewed by the IRB. |
Table 76 Protocol Document, Protocol Actions Page – Available Actions Descriptions