Notify IRB
Watch Video
- Protocol Training Video
Click arrows in the upper-left corner of video to navigate by section.
Reference Guides
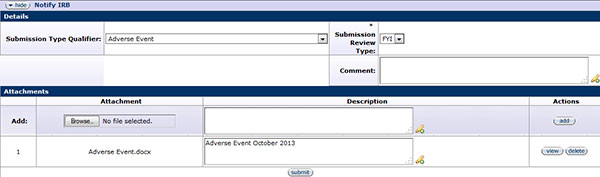
Figure 348 Protocol Document, Protocol Actions Page – Request an Action Section, Notify IRB
Utilize the Notify IRB action if you need to inform the IRB of an event or provide new information regarding an approved protocol, such as an adverse event, UPIRTSO, or deviation/exception/violation.
Actions |
Description |
|---|---|
Any untoward physical or psychological occurrence in a human subject participating in research. An AE can be any unfavorable or unintended event including abnormal lab finding, symptoms, or disease associated with the research or the use of a medical investigational test article. These must be reported to the IRB. |
|
Signficant New Finding |
PI must report any new knowledge or findings about medication or test article and/or study conditions that may develop. |
An exception is a circumstance in which a specific procedure called for in a protocol is not in the best interest of a specific subject (e.g., the subject is allergic to one of the medications provided as supportive care). Exceptions may not increase risk or decrease benefit, affect the participant's rights, safety, welfare, or affect the integrity of the resultant data. Exceptions must be reported to the IRB. Look for details on Emergency Exception, Enrollment Exception, Protocol Exception in the linked ORHP web page. |
|
A deviation is a violation that is unanticipated and happens without any prior agreement (e.g., a protocol visit scheduled outside of the protocol window). These must be reported to the IRB. Major deviations must be reported within five (5) working days. See the Reporting Events section of the linked OHRP web page. |
|
DSMB Report |
Data Safety Monitoring Board report. |
Other |
|
Self-Report for Non-Compliance |
Investigators and study staff are required to report instances of possible non-compliance. Use the Investigator Problem Report. |
The ORIC will investigate (if necessary), all complaints, concerns, and appeals received by the IRB. These include complaints/concerns from investigators, research participants, and others. Use the Participant/Researcher Complaint or Concern Form. |
|
Unanticipated problems involving risks to subjects or others (UPIRTSO) refers to any incident, experience, outcome, or new information that (1)is unexpected, (2) is related or possibly related to participation in the research, and (3) indicates that the subjects or other are at greater risk of harm (including physical, psychological, economic, or social) than was previously known or recognized. These must be reported to the IRB. |
Table 130 Protocol Document – Submission Type Qualifier Descriptions
In the Submission Review Type field, FYI is the default selection.
Attach a completed form for the corresponding action you are reporting in the Attachments section by clicking the browse button, providing a description, and clicking the add button. Links to the appropriate forms are found in Table Click the submit button to submit the action to the IRB.
History Section
Upon submission, the system will populate a notice to inform that the Notify IRB action was processed.
Figure 349 Protocol Document, Protocol Actions Page – Request an Action Section, Notify IRB notification
Additionally, the action being processed will display in the History section of your protocol with the date it was submitted and the ability to view the form attached. After the IRB has reviewed the activity report, it will acknowledge and provide a letter. The letter will be attached and can be viewed on the Notes & Attachments page.
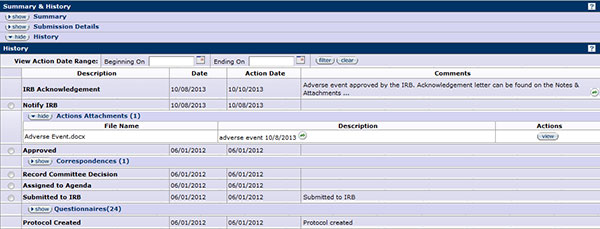
Figure 345 Protocol Document, Protocol Actions Page – Request an Action Section, Notify IRB, Summary & History Section