Other Identifiers
The Other Identifiers subsection provides the ability to attach additional identifiers to the protocol. If adding an additional identifier to the protocol, the type and specific number must be provided in order to add the information to the protocol. The approval date and comments fields are not required fields.
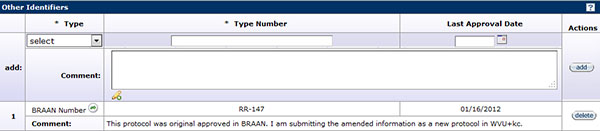
Figure147 Protocol Document, Protocol Page – Additional Information Section
To add an identifier to the protocol, click on the drop-down ![]() menu and select one of the options provided.
menu and select one of the options provided.
Enter the type number in the Type Number field by clicking within the text box and entering the appropriate information. To add the last approval date of that number (if applicable), click on the calendar ![]() icon to populate the desired date or enter the date within the text box in mm/dd/yyyy format. To add comments for each specific identifier, click within the text box and then type to enter text as necessary to provide the appropriate information. Once all fields have been completed, click the add
icon to populate the desired date or enter the date within the text box in mm/dd/yyyy format. To add comments for each specific identifier, click within the text box and then type to enter text as necessary to provide the appropriate information. Once all fields have been completed, click the add ![]() button to add the information to the protocol.
button to add the information to the protocol.
- Once added, an Other Identifier row may not be edited, but it can be deleted. To remove a line, click the delete
 button for the corresponding line.
button for the corresponding line.
| Other Identifiers | Description |
|---|---|
| CALGB | Cancer and Leukemia Group B |
| RTOG | Radiation Therapy Oncology Group |
| COAG | Clarification of Optimal Anticoagulation Through Genetics |
| ECOG-ACRIN | Eastern Cooperative Oncology Group |
| SWOG | Southwest Oncology Group |
| COG | Children's Oncology Group |
| NSABP | National Surgical Adjuvant Breast and Bowel Project |
| BRAAN NUMBER | BRAAN II Electronic Submission System number. After March 2015, all BRAAN protocols should have been transferred into WVU+kc. | Alliance | Alliance for Clinical Trials in Oncology | NRG | In 2014, RTOG, NSABP, and GOG joined together to form NRB Oncology. | CTSU | Cancer Trials Support Unit |
Table 57 Protocol Document, Protocol Page – Additional Information Section, Other Identifiers Descriptions
- For more information about basic line item functionality, see Common Line Item Operations in Common E-Doc Procedures.